|
|
Lab 1 - Overview of the Human Brain |
||
| Reading: | ||
| Coursera Media: | Perspectives on neuroanatomy Neuroanatomy is a complex subject. The wealth of anatomical detail discovered and described since the first systematic studies of human neuroanatomy began in the 16th century is simply staggering. With the constant introduction of powerful new neuroanatomical techniques, the advent of connectomics, and the U.S. federal BRAIN initiative, more details are arriving at an increasing rate, and there seems to be no end in sight. Fortunately, it is possible to acquire a rather simple (or simplified at least) anatomical framework for understanding the organization of the human brain and spinal cord, which is the foundation for understanding neurological functions and dysfunctions in clinical practice. Indeed, the neuroanatomical detail required for competent practice forms a very limited subset of the total information available. It is important not to lose sight of this reality as your knowledge grows and your understanding of the “Normal Body” matures. You will be challenged to build upon this simple neuroanatomical framework a more precise and accurate body of knowledge that will help you recognize various neurological impairments, injuries and diseases that afflict the nervous system; but first things first. Very soon you will encounter in hand—perhaps for the first time—the human brain. As we explore the basic parts of the human brain, don’t let the moment of excitement and wonder pass you by as you hold, closely inspect, and dissect what is widely declared to be the pinnacle of vertebrate evolution and the most complex structure in the universe! So let’s get ready and organized to make the most of our learning experiences in the laboratory setting. There are a few basic rules that you should follow in the laboratory when you examine human brains:
Experience encourages us to reinterate: please treat these specimens gently! Intact human brains are difficult to obtain, and with proper care, these specimens will be used for several years by various groups of learners. Lastly, you should recognize the incredible generosity of the individuals who donated their bodies (and brains) to biomedical research and the education of learners, such as yourselves. As you handle the brains in the lab, consider the courageous ambition of the donors and apply yourself to your learning accordingly. This will be one means of honoring the hopes and dreams of these anonymous (to us at least) individuals who exercised their final wish to advance human knowledge through their gift to you of discovery and learning.
How to use this Laboratory Guide Following this brief Introduction, you will find five chapters that address the foundations of clinical neuroanatomy that you will experience in the five corresponding lab sessions. These chapters address the basic embryological framework for understanding the human brain and the layout of the cerebral lobes (Lab 1); the functional organization of the cerebral cortex and the blood supply of the brain and spinal cord (Lab 2); the superficial features of the brainstem and spinal cord, including the cranial nerves, and a survey of internal features at representative levels (Lab 3); a more focused study of the internal features of the brainstem, with an emphasis on cranial nerve nuclei and important neuromodulatory nuclei (Lab 4); and, finally, the internal features of the forebrain (Lab 5). In addition, there are Appendices that highlight major somatic sensory, visual, and motor pathways, an overview of the limbic forebrain, and an orientation to problem-solving in clinical neuroanatomy. Application of this Laboratory Guide to your studies of the human central nervous system will provide the framework needed to understand neurological function and dysfunction, and to diagnose patients who are living with neurological injury, disorders and disease. So how should you approach your studies with this Laboratory Guide in hand? Here are some tips:
Given the pace and duration of the course, Brain & Behavior will come and go before you know it! We trust that these learning resources will serve you well now, and whenever a working knowledge of functional neuroanatomy is needed as you progress in your studies, scholarship and practice.
Lab 1 Overview
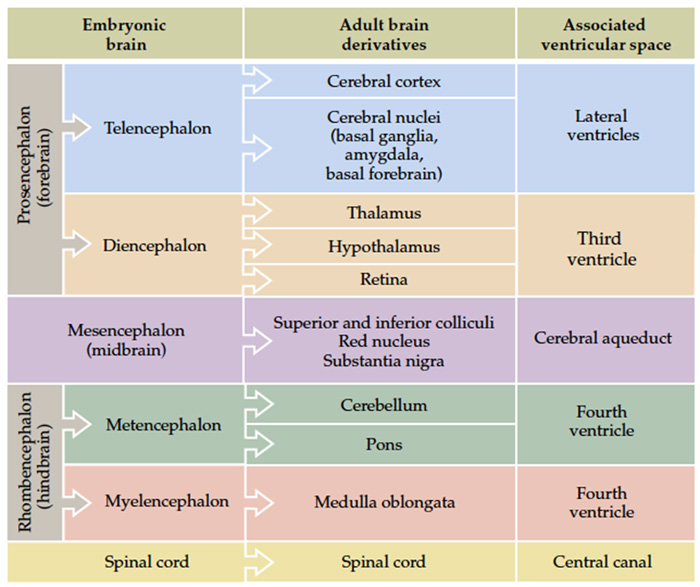
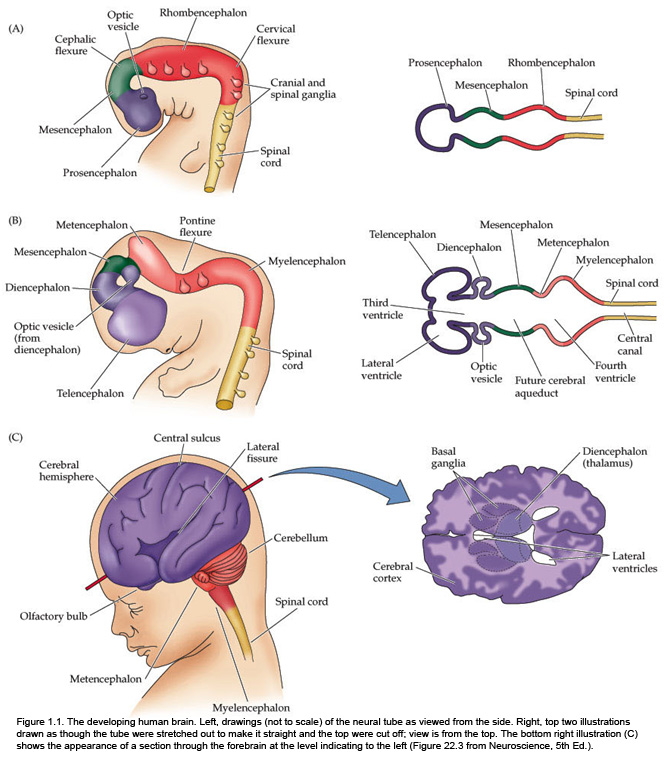
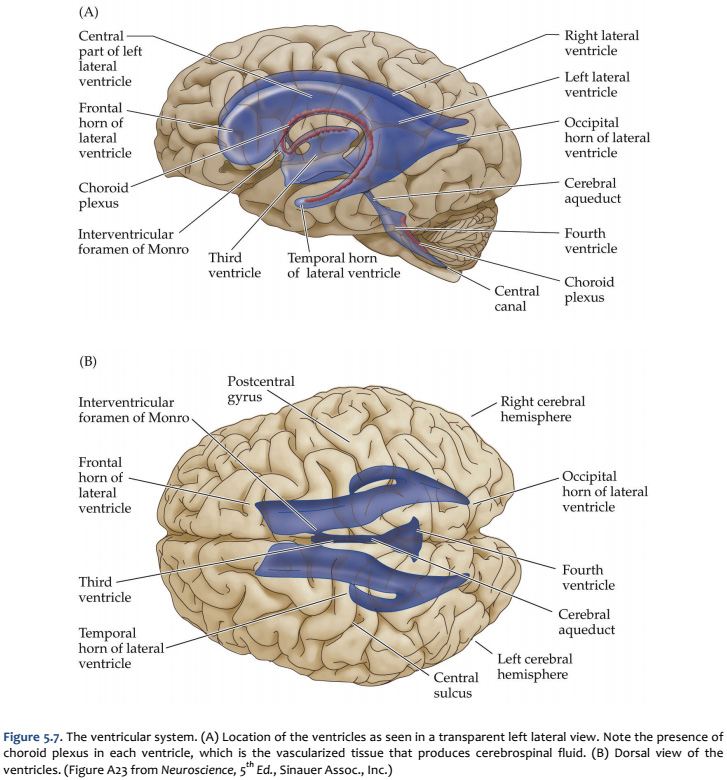
The fundamental divisions of the brain and spinal cord are easier to appreciate if you understand their embryological derivations. Thus, the human central nervous system (CNS)—as in all other vertebrate species—is derived from four basic embryological formations: the prosencephalon (forebrain), the mesencephalon (midbrain), the rhombencephalon (hindbrain), and the elongated spinal cord. The embryonic divisions of the CNS give rise to adult structures as summarized in the chart to the left. This chart depicts the conserved relationships among the parts of the developing brain and their adult brain derivatives, although the relatively greater growth of the cerebral hemispheres makes some of these relations somewhat difficult to appreciate (Figure 1.1). This first laboratory experience is designed to help you learn how to recognize major adult derivations of each embryological formation. Eventually, you will learn how to locate the components of important sensory, motor, and associational pathways in each subdivision of the central nervous system. So let’s begin our studies of human brain anatomy by recounting the basic events of neuroembryology. By the end of the first month of gestation, the neural tube closes and three swellings appear at its cephalic end (see Figure 1.1A). These will form the brain, while the rest of the neural tube gives rise to the spinal cord. The most rostral of the three, the prosencephalon (“forward brain” or “front brain”), soon divides into two parts: the telencephalon (“end brain” or “outer brain”), which gives rise to the cerebral hemispheres, and the diencephalon (“between brain” or “through brain”), which becomes the thalamus and hypothalamus. These structures together make up the adult forebrain. We will be using this term (forebrain) frequently, so its meaning in terms of brain subdivisions should become second-nature to you. Since the nervous system starts out as a simple tube, the lumen of the tube remains in the adult brain as a fluid-filled space. (Consider for a moment the fact that the entire brain is formed in the walls of a hollow tube!) This fluid-filled space, known as the ventricular system, is filled with cerebrospinal fluid (CSF) and provides an important landmark on images of the nervous system. As the brain grows, the shape of the central space also changes from that of a simple tube to its complex adult form (see Figure 1.2). The space, although continuous, takes different names in each of the subdivisions. Thus, the spaces inside the hemispheres are known as the lateral ventricles, and the space inside the diencephalon is the third ventricle. The mesencephalon, which is the middle swelling in the 4-week embryo (see Figure 1.1A),does not divide further and becomes the midbrain of the adult. The space inside the midbrain is called the cerebral aqueduct. It is important to appreciate the relationships among these structures in the adult brain so that you will understand why cross-sections through the brain in various planes appear as they do. The rhombencephalon further divides into the metencephalon, which becomes the pons and cerebellum, and the myelencephalon, which becomes the medulla (see Figure 1.1B). The neural tube caudal to these three cephalic swellings becomes the spinal cord. The space inside the developing rhombencephalon is called the fourth ventricle (see Figure 1.2). In early postnatal life, the development of the cerebellum gives rise to three apertures in the fourth ventricle that allow cerebrospinal fluid to exit the ventricular system and bathe the CNS in the subarachnoid space (see below). In the embryo and young children, the opening in the spinal cord is patent and is known as the central canal. However, it is very narrow and usually reduced to a “potential” space with very little CSF in the adult, with no continuous ventricular channel connecting the central canal to the fourth ventricle. Not all of the brain structures just mentioned (terms in bold) are illustrated in Figure 1.1. You will see all of them shortly as we explore the major parts of the adult brain. This will help to make relationships among brain structures and ventricular spaces in the adult much more obvious, especially in sectional views.
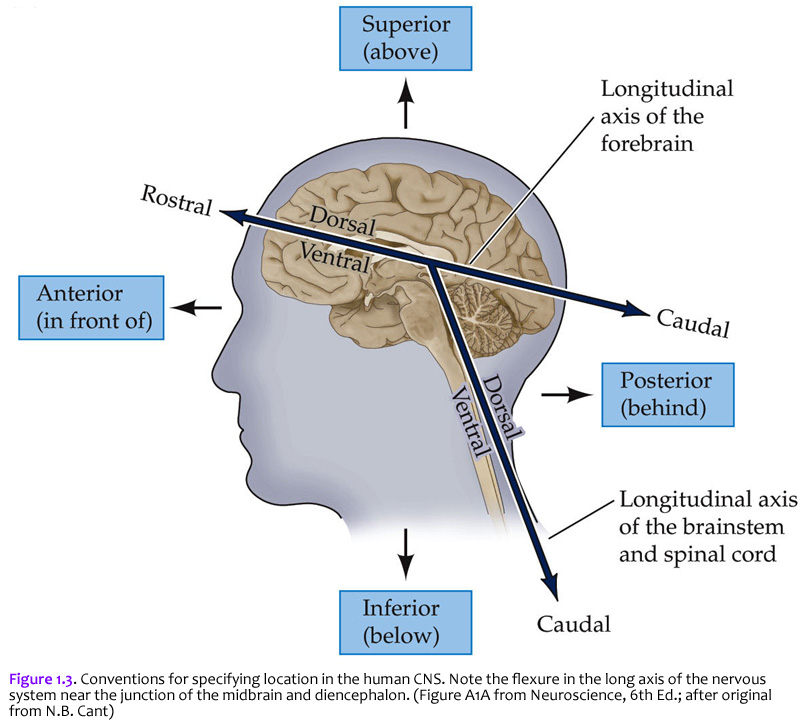
Now that you are reminded of the cellular components of nervous tissue in the central nervous system, we will return to the major theme of this first laboratory: understanding the basic plan of the human brain. But before examining the boundaries of the cerebral lobes in the telencephalon, it will be helpful to review several of the anatomical terms and conventions that are needed to specify position in the nervous system. The terms used to specify location in the central nervous system are the same as those used in gross vertebrate anatomy. One complication (that can become a source of confusion if you don’t understand it) arises because some terms refer to the long axis of the body, which is straight, and others refer to the long axis of the central nervous system, which has a bend in it (Figure 1.3). This bend – more properly termed, the cephalic flexure (see Figure 1.1A) – arose in the long axis of the nervous system as humans evolved upright posture. This flexure leads to a ~120 degree angle between the long axes of the hindbrain and forebrain. The two axes intersect at the junction of the midbrain and diencephalon. This flexure has consequences for the application of standard anatomical terms used to specify location. The terms anterior and posterior and superior and inferior are used with reference to the long axis of the body, which is straight. Therefore, these terms refer to the same direction in space for both the forebrain and the hindbrain (indicated by small arrows). In contrast, the terms dorsal and ventral and rostral and caudal are used with reference to the long axis of the nervous system, which bends. Thus, dorsal is toward the back for the hindbrain, but toward the top of the head for the forebrain. Ventral is toward the gut. Rostral is toward the top of the head for the hindbrain, but toward the “rostrum” (snout or front) for the forebrain; caudal is opposite (toward the back of the head for the forebrain or toward the tail for the hindbrain). When you understand these terms and how they are used, you will see why the terminology of neuroanatomy can be confusing at first. For example, the ventral aspect of the spinal cord is also referred to as the anterior aspect in humans, since for the human spinal cord, the two words are synonymous. However, there is a nucleus (cluster of neurons) in the thalamus called the “ventral anterior nucleus”. When reference is to the human forebrain, the two terms specify different directions, so the compound name of this nucleus is not redundant. Use of the terms discussed in this section allows us to specify the location of any part of the nervous system with reference to any other part. Figure 1.3 is worth some study of before proceeding.
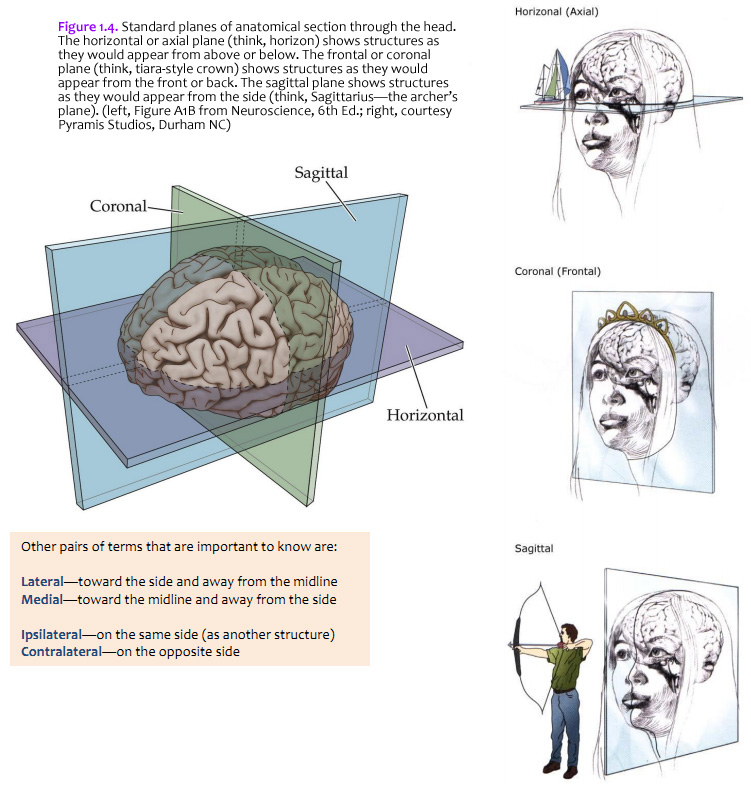
The brain is commonly cut in one of the three standard planes of section that you may be familiar with from your studies of gross anatomy (Figure 1.4). Magnetic resonance images (MRIs) are also usually made in these planes (or close approximations of them). It will help you to understand three-dimensional relationships in the brain if you become familiar with these planes, the application of the positional terms discussed in Figure 1.3, and the appearance of the internal structures of the brain in all three planes of section. Because of the cephalic flexure at the junction of the midbrain and diencephalon, coronal sections are the closest to cross-sections of the forebrain, whereas horizontal sections are the closest to cross-sections of the brainstem. (Cross-sections—also called transverse sections—are sections cut perpendicular to the long axis of the CNS.) Your first task when confronted with a new section of the brain is to figure out the plane of section. (Insets of the whole brain or brainstem are provided with most illustrations in this Guide.) Other pairs of terms that are important to know are: Lateral—toward the side and away from the midline Ipsilateral—on the same side (as another structure)
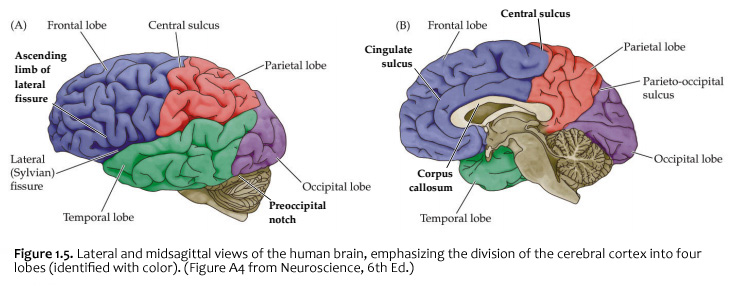
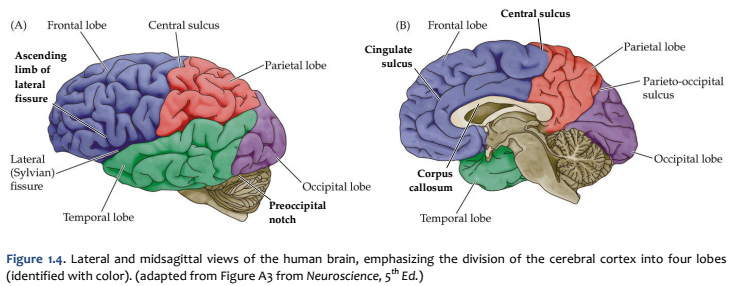
When you view the lateral aspect of a human brain specimen (Figure 1.5A; see also Grants 7.86A and Netters 99A), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem. The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane (Figure 1.5B). Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several sections and in Lab 2, you will find illustrations and photographs of these brain surfaces, and sufficient detail in the text to appreciate the overall organization of the parts of the brain that are visible from each perspective. As you work through this text during our first two laboratory sessions and in your studies following these experiences, you should find the structures and regions that are described here in Sylvius4 (to do so, launch Sylvius4 and go to Photographic Atlas, then select one of the atlas filters, such as Gyri, Lobes, or Sulci and Fissures). For now, we will focus on the landmarks of the cerebral hemispheres that have been used by convention to recognize the boundaries between adjacent lobes. Before delving into description of these landmarks, it is important to emphasize two important considerations:
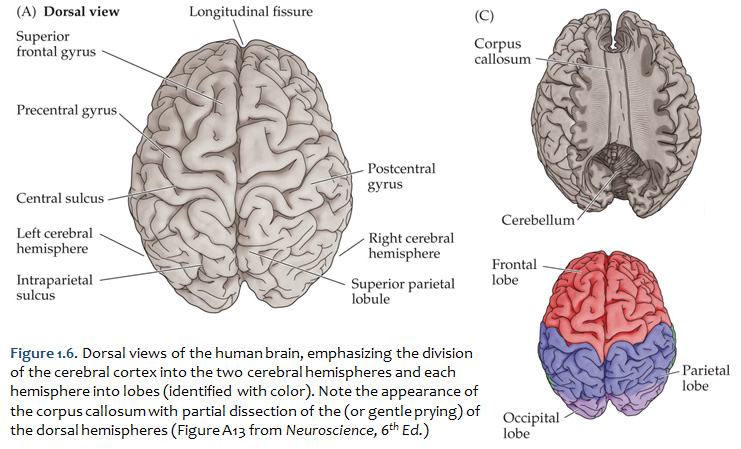
Concerning this first point, keep in mind the continuity of the cerebral cortex from one lobe to adjacent lobes. The boundaries between the lobes are merely surface features that reflect the highly infolded structure that developed in early life as the telencephalic vesicle expanded greatly to optimize its surface area within the closed compartment of the cranium. Thus, the cerebral hemispheres are especially large in humans. But the cerebral cortex itself is remarkably thin: it comprises a 3–5-mm thick layer of cells and cellular processes (after all, “cortex” means “bark” – like the relatively thin outer structure of a tree trunk). Concerning the second point, consider the fact that the human cerebral cortex is highly infolded. The ridges thus formed are known as gyri (singular: gyrus) and the valleys are called sulci (singular: sulcus) or fissures if they are especially deep. The appearance of the sulci and gyri varies somewhat from brain to brain. (As you might guess, each one has its own name, but it is necessary to become familiar with only a few of them). Nevertheless, all typically developed human brains have the same set of primary gyri, sulci and fissures in each cerebral hemisphere (the differences are mainly expressed in the size and shape of these primary features, as well in the myriad minor (secondary and tertiary) folds. A subset of these primary sulci and fissures are used by neuroanatomical convention to recognize the lobes of the cerebral hemispheres, which are named for the bones of the skull that overlie them, namely the frontal, parietal, occipital and temporal lobes (colorized in Figure 1.5). Despite these conventions, the surface landmarks that we use to recognize the boundaries between the lobes should NOT be taken to imply precise architectural or functional boundaries within the cerebral cortex. Arguably, the only cerebral boundary between adjacent lobes that bears such functional significance is the central sulcus (see below), which marks the boundary between the frontal and parietal lobes. It is conventional to assign motor functions to the cortex on the frontal lobe side of the central sulcus and somatic sensory functions to the cortex on the parietal lobe side. Contemporary neuroscience, however, is challenging the strict segregation of “motor” and “sensory” function and rather emphasizing the collaboration of frontal and parietal networks in somatic sensorimotor integration. For each of the other boundaries between adjacent lobes, which are clear enough as surface landmarks, the corresponding divisions of functional areas within the cerebral cortex are far less compelling. As the course progresses, we will explore in some depth the functions of the many areas of the cerebral cortex. For now, let’s focus on recognition of the surface features of the cerebral hemispheres that allow us to specify location in terms of cerebral lobes. Boundaries between lobes First, view the whole brain so that you are looking down from above (Figure 1.6). Notice the deep space that separates the left and right cerebral hemispheres. This space is called the longitudinal fissure (or superior sagittal fissure). As you will see as we explore the lateral and medial views of the hemispheres, the longitudinal fissure separates the corresponding frontal, parietal and occipital lobes of the two hemispheres. If you gently spread apart these lobes while looking down from above (very gently, please!), you should sight the massive white matter structure called the corpus callosum that conveys 100s of millions of axons from cortex in one cerebral hemisphere to the other. The frontal lobe is the most anterior of the four lobes and is separated from the parietal lobe by the central sulcus, which is one of the most important landmarks in the cerebral cortex (see Figure 1.5 and Figure 1.6, boundary between blue and red colored regions; see also pink box below). An important gyrus in the frontal lobe is the precentral gyrus. (The prefix ‘pre,’ when used to refer to anatomical position, refers to something that is in front of something else or that is anterior.) The cortex of the precentral gyrus is the somatic ‘motor cortex,’ which contains neurons whose axons project to the motor nuclei in the brainstem and spinal cord that innervate the striated muscles of the body. The cortex that forms the posterior bank of the central sulcus (in the parietal lobe) is the postcentral gyrus. (The prefix ‘post,’ when used to refer to anatomical position, refers to something that is behind something else or that is posterior.) The cortex of the postcentral gyrus is the ‘somatic sensory cortex’, which contains neurons that first receive incoming signals that originate in the somatic tissues of the body at the level of cortical processing. Thus, the banks of the central sulcus harbor the cortical representation of the contralateral half of the body for volitional movement and mechanical sensation.
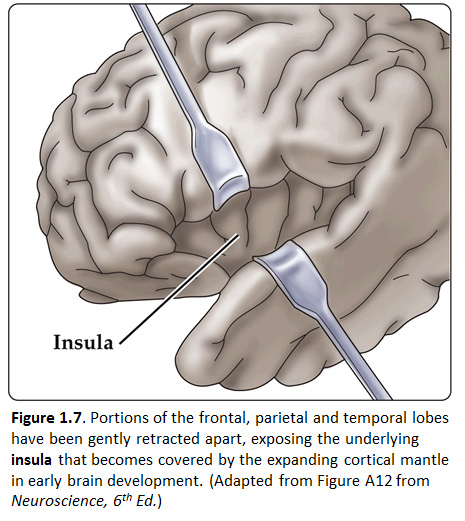
Now, view the lateral surface of either hemisphere near the lateral terminus of the central sulcus (see Figure 1.9). On the inferior-lateral aspect of the hemisphere, you should readily appreciate a deep and fairly straight fissure that separates the frontal and parietal lobes from the temporal lobe; this space is called the lateral fissure or Sylvian fissure (named after the important Renaissance neuroanatomist, Franciscus Sylvius, as was your digital brain atlas). Thus, temporal lobe is located inferior to the frontal and parietal lobes with the lateral fissure forming the superior margin of the temporal lobe. Turning our attention now to the posterior side of the lateral hemisphere, it is typically difficult to discern the boundaries where the posterior parietal and temporal lobes meet the anterior occipital lobe, and it may make no functional sense to attempt to do so. Nevertheless, we can define an imaginary lateral boundary between the parietal/temporal and occipital lobes in this complex and variable region of the human brain. This boundary is most arbitrary of them all and the one that is least well founded on underlying functional divisions from one region of the cerebral cortex to another. Nevertheless, it is neuroanatomical convention so we will localize this boundary. Emerging from the depths of the longitudinal fissure along the medial bank of the cerebral hemisphere (about one-fourth of the length of the fissure from its posterior limit) is the parieto-occipital sulcus (see Figure 1.5B). We will see this sulcus more plainly when we explore the medial face of the hemisphere (see below). For now, appreciate that the parieto-occipital sulcus is the boundary on the medial surface of the hemisphere between the parietal and occipital lobes. View the lateral surface of the hemisphere again and carefully inspect its inferior margin. Just above the cerebellum, there is often a small groove or notch in the gyral structure that can be appreciated 3-4 cm anterior from the caudal pole of the hemisphere. This groove is called the “pre-occipital notch” (see Figure 1.5A). Now, draw an imaginary line between the parieto-occipital sulcus dorsally and the pre-occipital notch ventrally; this line will serve as the lateral boundary between the parietal/temporal and occipital lobes. Now, let’s briefly turn our attention to a single cerebral hemisphere. When the brain is cut in the midsagittal plane, all of its subdivisions are visible on the cut surface (see Figure 1.5B). Just as in the embryo, the subdivisions are arranged as though they were stacked, with the cerebral hemisphere bulging out laterally at the top and the cerebellum bulging out dorsally and laterally about half-way up the stack. To recognize the medial boundary of the frontal and parietal lobes, locate again the medial terminus of the central sulcus (see Figure 1.5B). The gyral formation that surrounds the medial terminus of the central sulcus is called the paracentral lobule. Even on the medial face of the hemisphere, that sulcus marks the anterior boundary of the parietal lobe, at least its dorsal portion. The rest of the anterior boundary extends inferiorly through the paracentral lobule and then across the gyrus that follows the contour of the corpus callosum, which is called the cingulate gyrus. Now, find the parieto-occipital sulcus about halfway between the central sulcus and the posterior pole of the hemisphere. It should be present as a prominent sulcus running in nearly the coronal plane (actually, it is usually angled posteriorly from its inferior to superior ends) (see Figure 1.5B). You have already seen this sulcus from the dorsal view as it opens more widely into the longitudinal fissure. Seeing this sulcus in the midsagittal plane should now convince you that it is a prominent landmark that serves as the visible boundary between the parietal and occipital lobes. You can probably now appreciate why the boundaries of the occipital lobe are so much more definitive on the medial face of the hemisphere, as compared to the imaginary boundary on the lateral surface. Before we leave our consideration of the cerebral lobes from the lateral view of the brain, let’s again consider the fundamental fact from neuroembryology that the entire cerebral cortex in each hemisphere arose from the differential expansion and infolding of telencephalic vesicle. Consequently, you should not be surprised to discover that there is an important region of the cerebral cortex deep to the lateral (Sylvian) fissure that is completely hidden from view when examining the lateral surface of hemisphere. This region of cortex is called the insula. In early brain development, it became hidden beneath the expanding frontal, temporal, and parietal lobes. The components of these lobes that cover the insular cortex are often called ‘opercular’ components (‘opercular’ means a lid or cover). It can be seen if portions of these two lobes are retracted (as is illustrated in Figure 1.7). In spite of its name (one of the least helpful names in all of neuroanatomy), the insular cortex does not form an ‘island’. The insula is, roughly, the central portion of the continuous sheet of cortex and is deeply buried in the lateral fissure only because of the relatively greater growth of the cortex around it. Neuronal networks in the insular cortex are concerned with visceral, autonomic, and gustatory physiology and are thought to contribute in complex ways to integrative brain functions that impact emotion and social cognition. Indeed, “gut feelings” are believed to emanate from neural processing in the insula.
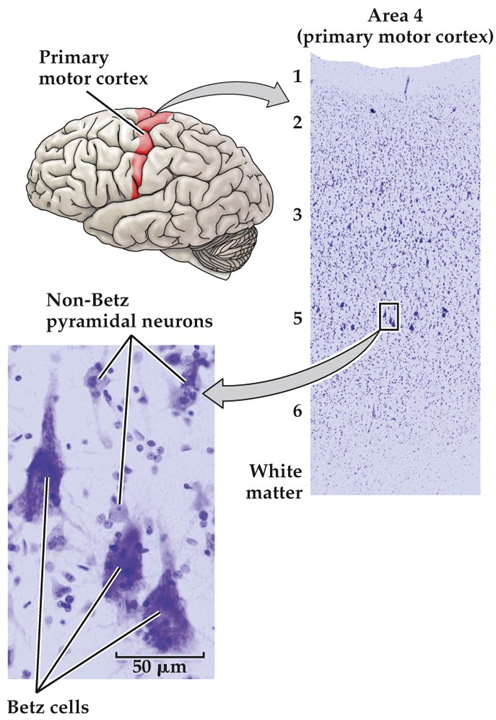
Early in the nineteenth century, the cell was recognized as the fundamental unit of all living organisms. It was not until well into the twentieth century, however, that neuroscientists agreed that nervous tissue, like all other organs, is made up of these fundamental units. The major reason was that the first generation of “modern” neurobiologists in the nineteenth century had difficulty resolving the unitary nature of nerve cells with the microscopes and cell staining techniques then available. The extraordinarily complex shapes and extensive branches of individual nerve cells—all of which are packed together and thus difficult to distinguish from one another—further obscured their resemblance to the geometrically simpler cells of other tissues (Figure 1.8). Some biologists of that era even concluded that each nerve cell was connected to its neighbors by protoplasmic links, forming a continuous nerve cell network, or reticulum (Latin, “net”). The Italian pathologist Camillo Golgi articulated and championed this “reticular theory” of nerve cell communication. Golgi made many important contributions to medical science, including identifying the cellular organelle eventually called the Golgi apparatus, the critically important cell staining technique that bears his name; and an understanding of the pathophysiology of malaria. His reticular theory of the nervous system, however, eventually fell from favor and was replaced by what came to be known as the “neuron doctrine.” The major proponents of the neuron doctrine were the Spanish neuroanatomist Santiago Ramón y Cajal and the British physiologist Charles Sherrington. The spirited debate occasioned by the contrasting views represented by Golgi and Cajal in the early twentieth century set the course of modern neuroscience. Based on light microscopic examination of nervous tissue stained with silver salts according to Golgi’s pioneering staining method, Cajal argued persuasively that nerve cells are discrete entities, and that they communicate with one another by means of specialized contacts that are not sites of continuity between cells. Sherrington, who had been working on the apparent transfer of electrical signals via reflex pathways, called these specialized contacts synapses. Despite the ultimate triumph of Cajal’s view over that of Golgi, both were awarded the 1906 Nobel Prize in Physiology or Medicine for their essential contributions to understanding the organization of the brain, and in 1932 Sherrington was likewise recognized for his contributions. The histological studies of Cajal, Golgi, and a host of successors led to the consensus that the cells of the nervous system can be divided into two broad categories: nerve cells, or neurons, and supporting glial cells (also called neuroglia, or simply glia). In contrast to nerve cells, glial cells support rather than generate electrical signals. They also serve additional functions in the developing and adult brain. Perhaps most important, glia are essential contributors to repair of the damaged nervous system, acting as stem cells in some brain regions, promoting regrowth of damaged neurons in regions where regeneration can usefully occur, and preventing regeneration in other regions where uncontrolled regrowth might do more harm than good. Neurons Neurons are distinguished by their specialization for intercellular communication and moment-to-moment electrical signaling. These attributes are apparent in their overall morphology, in the organization of their membrane components for long-distance signaling, and in the structural and functional intricacies of the synaptic contacts between neurons. The most obvious morphological sign of neuronal specialization for communication is the extensive branching of neurons. The two most salient aspects of this branching for typical nerve cells are the presence of an axon, and the elaborate arborization of dendrites that arise from the neuronal cell body in the form of dendritic branches (or dendritic processes; see Figure 1.9). Dendrites are the primary targets for synaptic input from the axon terminals of other neurons and are distinguished by their high content of ribosomes, as well as by specific cytoskeletal proteins. Some neurons lack dendrites altogether, while others have dendritic branches that rival the complexity of a mature tree (see Figure 1.8). The number of inputs a particular neuron receives depends on the complexity of its dendritic arbor: nerve cells that lack dendrites are innervated by just one or a few other nerve cells, whereas neurons with increasingly elaborate dendritic branches are innervated by a commensurately larger number of other neurons. The number of inputs to a single neuron reflects the degree of convergence, while the number of targets innervated by any one neuron represents its divergence. The information conveyed by synapses on the neuronal dendrites is integrated and “read out” at the origin of the axon, the portion of the nerve cell specialized for relaying electrical signals (see Figure 1.8 & 1.9). The axon is a unique extension from the neuronal cell body that may travel a few hundred micrometers or much farther, depending on the type of neuron (some axons in tall people can be a meter or more in length). The axon also has a distinct cytoskeleton whose elements are central for its functional integrity. Many nerve cells in the human brain have axons no more than a few millimeters long, and a few have no axons at all. Relatively short axons are a feature of local circuit neurons, or interneurons, throughout the brain. The axons of projection neurons, however, extend to distant targets over a range of millimeters to centimeters. The event that carries signals over such distances is a self-regenerating wave of electrical activity called an action potential, an all-or-nothing change in the electrical potential (voltage) across the nerve cell membrane that conveys information from one point to another in the nervous system. An action potential propagates from its point of initiation at the cell body (the axon hillock; see Figure 1.9B, asterisk) to the terminus of the axon, where synaptic contacts are made. The chemical and electrical processes by which the information encoded by action potentials is passed on at synaptic contacts to a target cell is called synaptic transmission. Presynaptic terminals (also called synaptic endings, axon terminals, or terminal boutons) and their postsynaptic specializations are typically chemical synapses, the most abundant type of synapse in the nervous system. Another type, the electrical synapse (facilitated by the gap junctions), is relatively rare and has special functions, such as inducing synchronous discharges among coupled neurons. Glial Cells Glial cells—usually referred to more simply as “glia”—are quite different from neurons. Glia do not participate directly in synaptic interactions or in electrical signaling, although their supportive functions help shape synaptic contacts and maintain the signaling abilities of neurons. Like nerve cells, glial cells have complex processes extending from their cell bodies, but these are generally less prominent and do not serve the same purposes as neuronal axons and dendrites. Cells with glial characteristics are the only apparent stem cells retained in the mature brain, and are capable of giving rise both to new glia as well as—in a few instances—new neurons. The word glia is Greek for “glue” and reflects the nineteenth-century presumption that these cells “held the nervous system together.” The term has survived despite the lack of any evidence that glial cells actually bind nerve cells together. Glial functions that are well established include maintaining the ionic milieu of nerve cells; modulating the rate of nerve signal propagation; modulating synaptic action by controlling the uptake and metabolism of neurotransmitters at or near the synaptic cleft; providing a scaffold for some aspects of neural development; and aiding (or in some instances impeding) recovery from neural injury. There are three types of differentiated glial cells within the gray and white matter tracts in the mature nervous system: astrocytes, oligodendrocytes, and microglial cells. Astrocytes, which are restricted to the central nervous system (i.e., the brain and spinal cord), have elaborate local processes that give these cells a star-like (“astral”) appearance (Figure 1.10A). A major function of astrocytes is to maintain, in a variety of ways, an appropriate chemical environment for neuronal signaling. In addition, recent observations suggest that a subset of astrocytes in the adult brain retain the characteristics of stem cells—that is, the capacity to enter mitosis and generate all of the cell classes found in the nervous tissue. Oligodendrocytes, which are also restricted to the central nervous system, lay down a laminated, lipid-rich wrapping called myelin around some, but not all, axons (Figure 1.10B). Myelin has important effects on the speed of the transmission of electrical signals. In the peripheral nervous system, the cells that provide myelin are called Schwann cells. In the mature nervous system, subsets of oligodendroglial cells and Schwann cells retain neural stem cell properties, and can generate new oligodendroglia and Schwann cells in response to injury or disease. Microglial cells are derived primarily from hematopoietic precursor cells (although some may be derived directly from neural precursor cells; Figure 1.10C). They share many properties with macrophages found in other tissues: they are primarily scavenger cells that remove cellular debris from sites of injury or normal cell turnover. In addition, microglia, like their macrophage counterparts, secrete signaling molecules—particularly a wide range of cytokines that are also produced by cells of the immune system—that can modulate local inflammation and influence cell survival or death. Following brain damage, the number of microglia at the site of injury increases dramatically. Some of these cells proliferate from microglia resident in the brain, while others come from macrophages that migrate to the injured area and enter the brain via local disruptions in the cerebral vasculature. An additional type of support cell associated with the central nercous system are ependymal cells, which which are simple columnar to cuboidal epithelial cells that line the fluid-filled ventricles and form the choroid plexus of the lateral ventricle and the fourth ventricle, where a subset of highly specialized ependymal cells express membrane associated Na/K ATPases and aquaporins to produce cerebrospinal fluid. Cellular Diversity in the Nervous System Although the cellular constituents of the human nervous system are in many ways similar to those of other organs, they are unusual in their extraordinary numbers. The human brain is estimated to contain on average about 85 billion neurons and about the same number of glia cells. More important, the nervous system has a greater range of distinct cell types—whether categorized by morphology, molecular identity, or physiological role—than any other organ system. The cellular diversity of any nervous system undoubtedly underlies the capacity of the system to form increasingly complicated networks and to mediate increasingly sophisticated behaviors. Consider, for example, the appearance of just one division of the cerebral cortex (primary motor cortex in the precentral gyrus) stained to demonstrate the distribution of all cell bodies—but not their processes or connections—in neural tissue (Figure 1.11). This widely used Nissl method stains the nucleolus and other structures (e.g., ribosomes) where DNA or RNA is found. In the cerebral cortex, cells are arranged in layers, with each layer defined by distinctive differences in cell density. Virtual microscopic review of the central nervous system A. Lumbar Spinal Cord - Webslide 0302_O (cat): Spinal cord, c.s., Toluidine Blue [ImageScope] [WebScope]
B. Thoracic Spinal Cord - Slide 66a (thoracic spinal cord, luxol blue & cresyl violet) [ImageScope] [WebScope]
C. Glial Cells
D. Cerebellum - Webslide 0085_C, cat, LFB-CFV [ImageScope] [WebScope]
E. Neocortex, Cerebrum - Slide 76 (cerebrum, luxol blue/cresyl violet) [ImageScope] [WebScope] & Slide 76b (toluidine blue & eosin) [ImageScope] [WebScope]
F. Archicortex, Hippocampal Region - Slide NP004N (hippocampal region, coronal section, luxol blue) [ImageScope] [WebScope] [ORIENTATION]
|
|
Click here to submit questions or comments about this site. Duke University | Duke Medicine | School of Medicine | School of Nursing | Doctor of Physical Therapy Updated 12/28/17- Velkey |
||